The Team
Myriam Heiman, PhD is an assistant professor at the Massachusetts Institute of Technology (MIT) in the Department of Brain and Cognitive Sciences. She is also a principle investigator at MIT’s Picower Institute for Learning and Memory and a core member of the Broad Institute of MIT and Harvard.
Dr. Heiman joined MIT in 2011 and combines genetics, biochemistry, cell biology, and neuroscience to tackle the problems of cell identity and vulnerability in the diverse environments of the nervous system. She is known world-wide for developing technologies and methods that enable specific and large-scale analyses of particular types of cells at particular times during normal function and in diseased states. Some of these tools include genetically engineered TRAP mice, and the new SLIC methodology.
Her current focus is to apply her approaches to understand brain illnesses such as psychiatric and neurodegenerative disease. Prior to joining MIT, Dr. Heiman was a postdoctoral researcher with Dr. Paul Greengard at The Rockefeller University. Her scientific training started at Princeton University, where she received her B.A. in molecular biology and continued on at Johns Hopkins University to earn a Ph.D. in biology.
Her work has been published in top-tier scientific journals like Cell and Nature and recognized by several awards, including an NIH/NINDS EUREKA award, and a William N. and Bernice E. Bumpus Foundation Innovation Award, given to promising, early-career investigators.
Amanda Vernon is a graduate student at the Massachusetts Institute of Technology (MIT) in the Department of Brain and Cognitive Sciences. She is a member of Myriam Heiman’s laboratory, which works at the molecular and cellular levels to improve understanding of neuronal identities and specific vulnerabilities, especially in the context of neurological diseases.
Amanda’s current work is focused on improving our understanding of the cellular abnormalities underlying schizophrenia by elucidating the molecular and cellular effects that chronic antipsychotic treatments have in brain regions most relevant to schizophrenia.
Previously, Amanda worked in the lab of Tyler Jacks, director of the Koch Institute for Integrative Cancer Research, at MIT. In this capacity, she worked to characterizing a novel mouse model of anaplastic thyroid cancer.
Amanda received her Bachelor of Science degree and Honors in Neuroscience from Brown University in 2012. Amanda completed her undergraduate thesis work in the lab of Gideon Koren, director of the Cardiovascular Research Center at Brown and Rhode Island Hospital, towards a better understanding of cellular mechanisms underlying Long QT Syndrome.
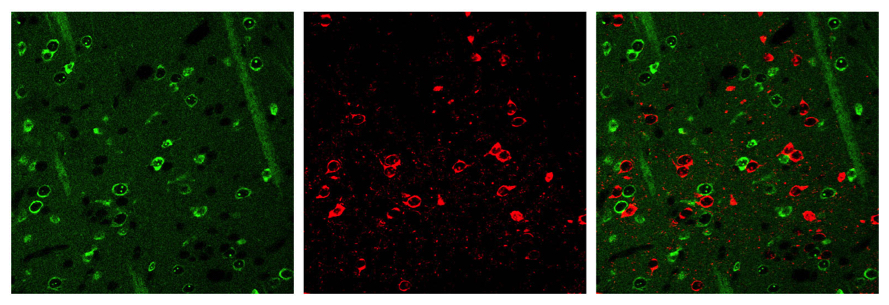
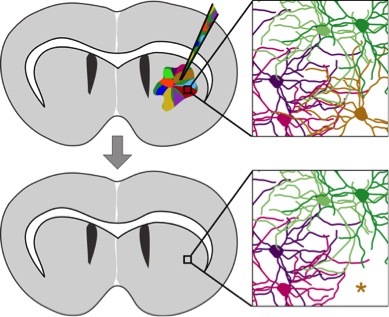
The Heiman Lab aims to understand how neuronal identity is established and maintained, and how the molecular identity of a neuron determines its susceptibility to disease. Lab members use biochemical, genetic, and molecular biological tools, including mouse models of brain diseases and novel methodologies termed Translating Ribosome Affinity Purification (TRAP) that allows molecular profiling of individual types of neurons from within the mammalian brain and Synthetic Lethal in the Central Nervous System (SLIC), which makes it possible to screen thousands of genes in the central nervous system.
Many diseases and disorders of the nervous system affect only particular groups of cells. Understanding the cause of enhanced vulnerability to disease displayed by the affected cell groups in each case may point toward the root causes of these diseases and disorders, and will likely lead to novel therapeutic targets.
One of Dr. Heiman's inventions is a breakthrough cellular analysis that solves a problem that has perplexed neurological researchers for decades. Heiman helped to develop a method that reveals the kinds and amounts of proteins different cells produce, what biologists call the cell's translational profile. The technique involves isolating the genetic messages that govern protein production in different cell types. The method, "translating ribosome affinity purification" (TRAP), can identify all the genetic messages that give a cell type its unique identity, including perhaps its susceptibility to disease. The true breakthrough lies in its ability to distinguish the profile of any cell type in any tissue in the body. Its usefulness is not just limited to brain cells, meaning it has far-reaching research applications -- cancer, heart disease, diabetes, as well as many others. Myriam Heiman employs the new technique to study the biochemical bases of brain and development diseases. It is also being applied to the still-mysterious ways in which psychoactive drugs fight schizophrenia.
The TRAP tool advances the speed at which researchers can obtain cell type-specific results and should fundamentally change biochemical studies of the brain. “We can look at thousands of genes instead of one at a time, giving us a genome-wide perspective of a cell’s translatome,” says Dr. Heiman. This new technique will help accelerate scientific research into discovering the subtle molecular differences amongst the hundreds of specialized cell types. A deeper understanding of body cell mechanisms will help researchers investigate the causes of brain illness and disease.
More recently, Myriam Heiman has developed a methodology for identifying genetic modifiers of disease called SLIC (Synthetic Lethal in the Central Nervous System). The method makes it possible to screen thousands of genes in the central nervous system to identify those that make cells most or least vulnerable to disease. In vitro screens — performed in a culture dish — can’t always pinpoint these genes. But the new method uses screens performed in the actual cell type affected to let researchers identify genetic modifiers of disease progression. The result is an unbiased view of how all genes in the genome can affect disease progression.